
“Substantial and sustained” decline in chlamydia and syphilis among Doxy PEP users in Northern California
Two studies published in JAMA Internal Medicine give evidence about Doxy PEP's "real world" effectiveness in preventing STIs.
We conduct and publish original research that provides deeper understandings of our communities, driving forward innovations in health, substance use treatment and HIV prevention. We partner with researchers both locally and nationally to address gaps in knowledge regarding how HIV affects our communities.
Research Staff: Hyman Scott (Chief Medical Officer) and Kelvin Moore (Clinical Research Coordinator)
Support Staff: Jorge Roman (Senior Director, Clinical Services), Jasmin Alvarez (Director of Clinical Operations), Jorge Hernandez (Associate Director of PrEP & HIV Positive Services), Fatima Ticas (Manager of Laboratory Service), Rikki Montoya (Medical Assistant, Clinical Research).
There exist many barriers to taking daily, oral PrEP. Numerous studies have shown that the efficacy of PrEP is highly dependent on daily use and adherence. The PCORI PrEP Study is a clinic-based, multi-site, randomized, two-arm study to compare the effectiveness of two mobile technologies designed to support PrEP adherence and continuation. Participants are followed throughout the course of a year and their study visits coincide with the health visit at the Strut sexual health and wellness center. Compensation for this study is $75 per visit with 3 visits. There are additional study activities for which participants are compensated.
Eligibility requirements:
Investigators at the San Francisco AIDS Foundation and the University of California San Francisco are developing a novel point-of-care (POC) test to measure urine drug-levels to PrEP (Truvada only) for the first time, providing the opportunity to target and enhance adherence counseling during a routine clinical visit. This study involves collecting a one-time urine specimen to measure PrEP drug levels and administering a one-time survey which will ask questions about PrEP use, sexual behavior, motivators to use PrEP, and the impact of the COVID-19 epidemic on PrEP use. Compensation for this study is $50.
Eligibility requirements:
In 2018, San Francisco AIDS Foundation programs including the Elizabeth Taylor 50-Plus Network, TransLife and Programa Latino recruited participants in collaboration with ACRIA for the ROAH Study. The ROAH study examined the health and wellness of older adults living with HIV in our community, and found that the majority of people described their physical health as excellent or good, nearly all participants were receiving antiretroviral therapy, and over 90% reported having an undetectable viral load.
The DISCOVER study is a clinical trial evaluating the safety and efficacy of Descovy (a candidate PrEP medication) to prevent HIV in men who have sex with men and transgender women. The study enrolled 5,400 participants in the United States, Canada and Western Europe. The study demonstrated that Descovy is effective for HIV prevention with non-inferiority to Truvada and that Descovy has an improved renal safety profile for those who have kidney-associated complications.
Current: In partnership with San Francisco State University (SFSU), an applied research project to assess bio-psycho-social needs of Spanish Speaking Communities living on the San Francisco Streets
Past: In partnership with SFSU and UC Berkeley (UCB), an applied research project to assess the ability of rural immigrants to receive HIV testing and counseling, PrEP information and counseling, HIV care and support or mental health services in rural California
Past: In partnerships with SFSU and UCB, an applied research project to assess and identify health challenges including the prevalence of illness, point of entry to health care, retention and continuum of treatment (in the United States, and /or Mexico or cyclic transits), and their use of the Affordable Care Act services.
The STEP Study was conducted with participants in the substance use counseling program of SFAF (The Stonewall Project), to determine how The Stonewall Project changed meth use, sexual risk-taking behavior and HIV health. Participants reported reductions in methamphetamine use and sexual risk-taking behavior while using methamphetamine. The Stonewall Project is a harm reduction-based substance use treatment program of San Francisco AIDS Foundation that provides services to gay/bi and other men who have sex with men.
PACE was designed to determine the impact of several strategies to reduce Blood Alcohol Concentration (BAC) among gay bar patrons in San Francisco. The study revealed that media campaigns, normative feedback and structural interventions such as free water in bars were able to reduce BAC by 30%.
Up to 2018, PrEP clinical studies had largely excluded trans people as study participants, leaving unanswered questions about the effects of PrEP on gender-affirming hormone therapy and vice versa. To study this question, SFAF, in collaboration with UCSF, conducted a study with trans people taking gender-affirming hormones and PrEP. Results are pending.
The steps of the HIV care cascade often function as an obstacle course for individuals who have been newly diagnosed with HIV. A new HIV diagnosis must be confirmed, usually by methods that take several days, and then individuals must link to medical care for screening labs and antiretroviral therapy (ART) initiation, a process that often requires insurance enrollment or changes. These steps can be time-consuming and difficult to navigate logistically and emotionally.
The RAPID approach, pioneered at UCSF’s Ward 86 HIV clinic, seeks to collapse the many steps of the care cascade and simplify the process of ART initiation. In the RAPID model, patients receive immediate ART, including through starter packs, even as other aspects of their care are being arranged. The Ward 86 model began through a system of same or next-day referrals from testing sites around San Francisco. The “Test and Be Treated” or TBT study, supported by the California HIV Research Program, seeks to streamline the process of ART initiation even further.

Two studies published in JAMA Internal Medicine give evidence about Doxy PEP's "real world" effectiveness in preventing STIs.

Twice-yearly lenacapavir reduced the risk of HIV acquisition by 96% among gay and bisexual men and transgender and nonbinary people.
From lenacapavir offering 100% protection against HIV among cis women, to the potential for long-acting PrEP to end the HIV epidemic, Liz Highleyman offers a thorough overview of the current state of injectable PrEP.
For the first time, research demonstrates that doxycycline taken daily reduces STI incidence by 67% in cisgender women. Gus Cairns explains the science, and shares why previous studies may have failed.
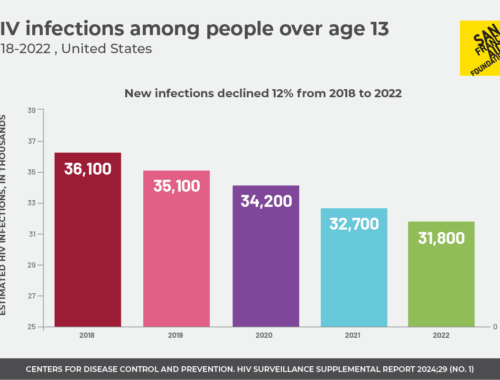
Infections across the nation decreased overall from 2018 to 2022, although disparities remain.

SFAF y la Coalición Nacional para la reducción de daños llevó a cabo una evaluación de necesidades para analizar las condiciones, los retos, y las barreras que afectan a las personas latinas que consumen drogas.

Download a fact sheet highlighting excerpts from peer-reviewed research on the effects of criminalization of drug use.

Here’s how you can participate in a study to test the vaccine’s effectiveness.