Islatravir: A potential PrEP & HIV drug now being tested
There’s a new antiretroviral, from a new class of medication, currently being tested for both PrEP and HIV treatment. Named islatravir (formerly MK-8591), this Merck drug is showing promise as part of a novel drug delivery system and because of its long half-life, great potency and high barrier for resistance.
Two presentations at the IAS 2019 conference in July shared Phase 2 study data on islatravir. Randolph Matthews, MD, PhD shared research of islatravir delivered through an under-the-skin implant, to test drug delivery at concentrations needed for the prevention of HIV. Professor Jean-Michel Molina, from Paris Diderot University, shared results from a dose-ranging study testing three different doses of islatravir, in combination with doravirine, to suppress viral loads in treatment-naïve adults living with HIV.
“It is clear that PrEP and HIV medication alternatives are evolving,” said Christopher Hall, MD, MS, AAHIVS, vice president of medical affairs at San Francisco AIDS Foundation. “More options are needed especially for PrEP, because a single agent will never meet the needs of all people. Personal choice certainly will take on greater significance as alternatives evolve.”
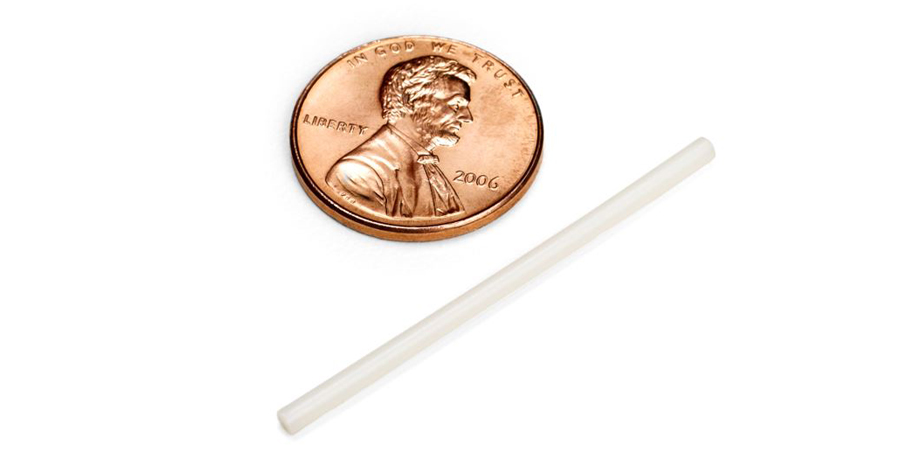
Islatravir in a PrEP implant
Based on a design similar to that used for the Nexplanon/Implanon birth control implant, the islatravir implant is a removable implant designed to be inserted under the skin in the upper arm for a year. In the study presented by Matthews, researchers tested the drug concentration levels delivered by the islatravir implant over 12 weeks in 16 individuals.

In a double-blind, placebo-controlled study, the researchers tested two doses of the islatravir implant: 54 mg and 62 mg. Throughout the study, the researchers collected drug concentration levels in plasma and PBMCs, and were primarily interested in how much time the drug concentration levels fell below a threshold of 0.5 pmol/106 (a threshold which is believed to provide an adequate level of drug for HIV prevention based on animal studies).
At both doses, drug concentrations remained above the 0.5 pmol/106 threshold for the 12 weeks of the study, although the lower limit of the estimated range for the 54 mg dose dipped below the threshold for a brief period. The 62 mg implant delivered drug concentration levels well above the threshold for the duration of the study.
In a later analysis, the researchers projected that the 62 mg implant would lead to concentrations above the threshold for at least 12 months, falling below the threshold at around 16 months.
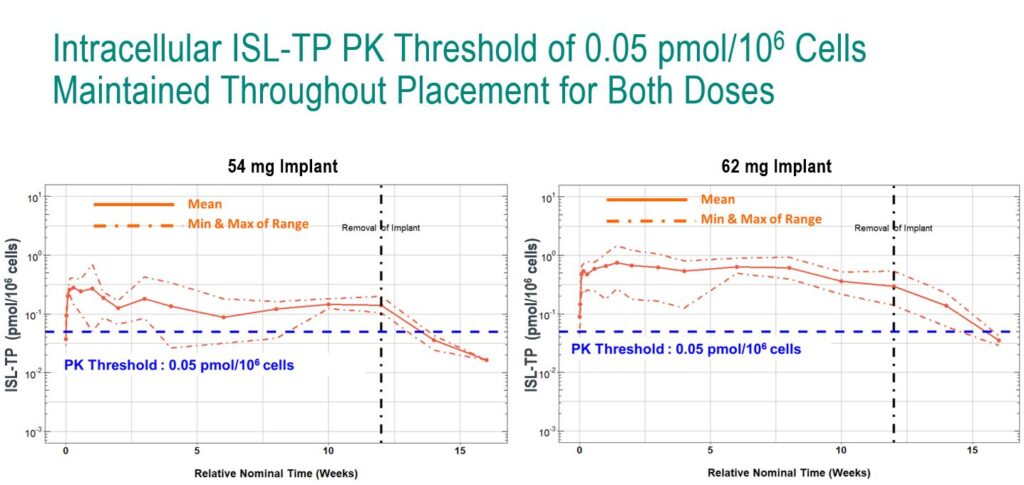
“This supports the potential of the islatravir implant as a once-yearly PrEP option,” said Matthews.
Future research, he said, will continue to test other prototype implants and additional doses.
“Just as in birth control, implanted, long-acting pharmaceuticals are likely to earn a place in the biomedical HIV prevention armamentarium,” said Hall. “While more study is needed, clearly the future of PrEP is one of greater alternatives, with agents that bring fewer side effects, are less susceptible to resistance, are easier to administer, and improve the experience of people who take them. The future promises a broader array of options than we have seen since the advent of FDA-approved Truvada for PrEP in 2012, and it is bright.”
Islatravir for dual-therapy HIV treatment
There are a few unique characteristics of islatravir that may make it suitable as part of a two-drug regimen, said Molina. In preclinical studies, islatravir showed more than 10-fold greater potency compared to other approved antiretrovirals. The drug is also known to act against a number of resistant HIV variants, has a long half-life and has a high barrier to resistance.
“This [two-drug regimen] may maintain efficacy comparable to a three-drug regimen,” said Molina.
To test this, a double-blind study involving 120 treatment naïve adults living with HIV randomized participants to receive islatravir 0.25 mg, 0.75 mg, 2.25 mg or doravirine plus 3TC (lamivudine)/TDF (tenofovir disoproxil fumarate). At 24 weeks, or once viral loads were suppressed to <50 copies/mL on the triple-therapy, 3TC was removed.
There were very high response rates to the dual therapy at every dose level of islatravir.
After 24 weeks on the dual therapy, close to 90% of people receiving either islatravir 0.25, 0.75 and 2.25 mg with doravirine had viral loads <50 copies/mL. (96% of people receiving the control treatment of doravirine, 3TC and TDF had viral loads suppressed to this level.)
There were very few adverse events during the study, with two out of 90 participants receiving islatravir discontinuing the study because of adverse events.
“These results are quite promising, and demonstrate that the dual combination of islatravir and doravirine has the potential to be a potent, two-drug regimen and supports its further exploration in Phase 3 trials,” said Molina.
More research on islatravir’s safety and efficacy is needed before the drug may be granted FDA approval and brought to market. Based on the results of these studies, Merck plans to continue Phase 3 research on islatravir for HIV treatment as part of a once-daily, oral two-drug regimen. Using islatravir for PrEP, Merck is pursuing a Phase 2 study, set to begin enrolling in September 2019, that will evaluate the safety and tolerability of once-monthly oral islatravir.
Sources:
Matthews, R. and colleagues. First-in-human trial of MK-8591-eluting implants demonstrates concentrations suitable for HIV prophylaxis for at least one year. IAS 2019, Abstract TUAC0401LB.
Molina, J. and colleagues. MK-8591 at doses of 0.25 to 2.25 mg QD, in combination with doravirine establishes and maintains viral suppression through 48 weeks in treatment-naïve adults with HIV-1 infection. IAS 2019, Abstract WEAB0402LB.