Digging into the science–and promise–of long-acting injectable PrEP
Long-acting pre-exposure prophylaxis (PrEP) has the potential to change the landscape of HIV prevention. Daily oral PrEP is highly effective, but taking a pill a day isn’t for everyone. Long-acting injectable PrEP can now be given every other month, and twice-yearly shots are on the horizon. But long-acting PrEP can only change the game if the people who need it most have equitable access.
By now, the benefits of daily oral PrEP are well known. Tenofovir disoproxil fumarate/emtricitabine (TDF/FTC; Truvada) is around 99% effective for gay and bisexual men and transgender women if taken consistently. Taking TDF/FTC before and after sex also works well. The newer tenofovir alafenamide/emtricitabine (TAF/FTC; Descovy), is as effective as TDF/FTC for people who are not exposed to HIV via vaginal sex. Studies have found that PrEP pills seem to be less effective for cisgender women, but this is mainly due to suboptimal adherence.
Long-acting cabotegravir (ViiV Healthcare’s Apretude) was approved for PrEP in 2022. Two large studies showed that Apretude injections administered once every other month are even more effective than daily TDF/FTC for both men and women. And at the International AIDS Conference last month in Munich, researchers reported that a relatively new antiretroviral, Gilead Sciences’ lenacapavir, provides very good protection when given once every six months.
In the PURPOSE 1 trial, lenacapavir showed 100% efficacy for young cisgender women in South Africa and Uganda. None of the 2,134 women randomly assigned to receive twice-yearly lenacapavir injections acquired HIV, while there were 39 new infections among the 2,136 women who received TAF/FTC and 16 among the 1,068 participants assigned to TDF/FTC. The injections worked better than the daily pills, which were hampered by poor adherence. In contrast, adherence to lenacapavir was high, with more than 90% of participants receiving their shots on time.
“These stellar results show that twice-yearly lenacapavir for PrEP, if approved, could offer a highly effective, tolerable and discreet choice that could potentially improve PrEP uptake and persistence, helping us to reduce HIV in cisgender women globally,” said Dr. Linda-Gail Bekker of the Desmond Tutu HIV Centre at the University of Cape Town, who received a standing ovation after her presentation.

A parallel trial of twice-yearly lenacapavir PrEP for gay and bisexual men, transgender women and men and nonbinary people, dubbed PURPOSE 2, is currently ongoing and results are expected by late this year or early 2025. Trials for cisgender women in the United States (PURPOSE 3) and people who inject drugs (PURPOSE 4) are also underway.
Like other antiretrovirals, lenacapavir stops HIV replication. It is not a vaccine that trains the immune system to fight the virus. But after four decades of effort, there are still no effective HIV vaccines, and twice-yearly PrEP could be the next best thing. According to Centers for Disease Control and Prevention, there were 31,800 new HIV infections in the United States in 2022, and only around a third of people who could benefit from PrEP have gotten a prescription.
Further back in the pipeline, researchers are exploring other long-acting prevention tools, including broadly neutralizing antibodies and implants that could potentially offer even longer-lasting protection.
“Any technology that adds another option for folks is a good thing,” says Jorge Roman, MSN, FNP-BC, AAHIVS, San Francisco AIDS Foundation’s senior director of clinical services. “I think if we can get to the day when we have pills, we have injectables, we have an implant that lasts for a year or more, that will do so much for our ability to end the HIV epidemic.”
Injectable Pros and Cons
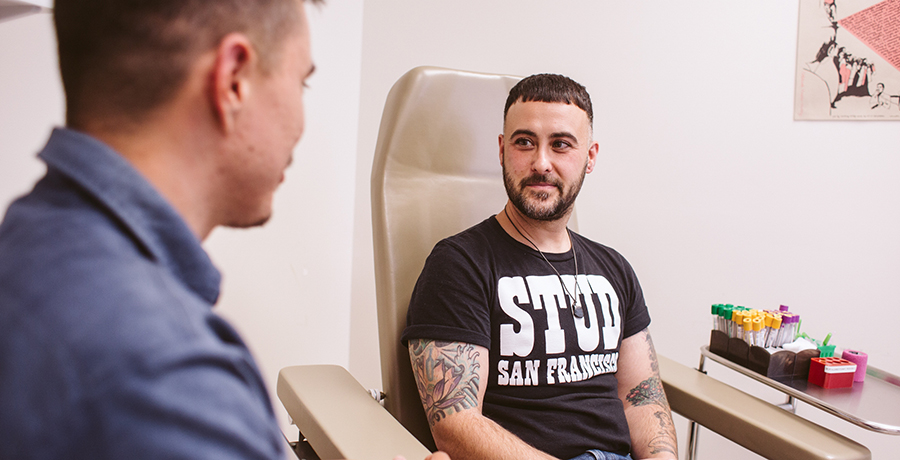
PrEP pills have been eagerly adopted by urban white gay men, but uptake has been slower among Black and Latino gay men and cisgender women of all racial and ethnic groups. Use of long-acting injectable PrEP remains low in the United States, and access is still limited worldwide.
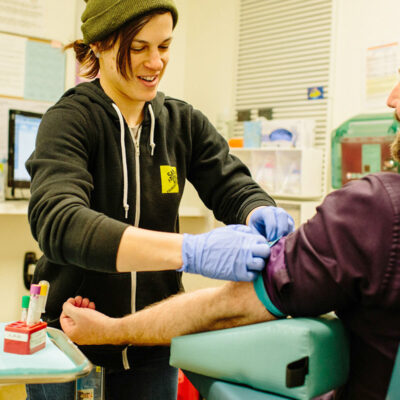
At SFAF’s Magnet clinic and auxiliary sites, only around 75 to 100 of the 2,700 PrEP clients have opted for Apretude, according to Roman.
There are a number of reasons why people might prefer long-acting injectables. “Folks like the simplicity of not having to remember to take a daily pill,” Roman told BETA. “I think that’s the most common reason: ease of use.”
Others are hesitant to have pill bottles that could be lost or stolen or reveal that they’re at risk for HIV. Concerns about stigma and even violence can be a barrier, especially for young women. A recent study showed that injectable PrEP could be a good option for people with unstable housing and substance use issues, especially if they receive support to make sure they get their shots on time.
On the other hand, people who prefer pills cite the inconvenience of regular clinic visits. A client who opts for Apretude will need to come in seven times during the first year, compared with four times for those who choose daily PrEP. And some simply dislike injections, which for Apretude involves a rather large-volume shot in the butt.
“Some people don’t like needles. They’ve heard from some of their friends that the injections can be pretty painful, and the pain can last a little longer than your run of the mill vaccine,” Roman said. “Some people don’t like the fact that it’s a newer medication that has not been around as long as oral PrEP, and we have a lot more data for both Truvada and Descovy. They’re just not comfortable.”
Side effects can also differ. People taking PrEP pills may experience nausea, and some are concerned about kidney problems and bone loss associated with TDF/FTC, Roman noted. (TAF/FTC is easier on the kidneys and bones.) PrEP shots, on the other hand, can cause injection site reactions and nodules under the skin.
Another concern with long-acting injectables is the so-called “long tail.” Because Apretude takes a long time to leave the body, people need to use another form of protection after stopping the shots so that if they do acquire HIV, the virus won’t be exposed to low drug levels and develop resistance.
“Once they stop the injectable, we counsel people that they’ll need to stay on some HIV prevention method for up to a year afterwards,” Roman said. “Often a reason why people won’t choose the injectable is knowing that if they do stop it eventually, they they’re going to have to go back to oral PrEP anyway.”
There are also challenges on the provider side. In an era of shrinking health resources, clinic space and doctors’ and nurses’ staff time are at a premium.
“Administratively, there’s a lot of work involved in getting people on Apretude,” including the benefits navigation aspect, Roman said. “Just the person power is a big part of that, and also the amount of space and the amount of clinic time it takes. For that reason, we have decided, at least until we catch up with ourselves, to limit ourselves to 100 clients on Apretude until we feel like we’re at a good pace.”
Cost and insurance coverage can also be a barrier. Apretude costs around $4,000 per shot or $24,000 per year. Insurers are required by law to fully cover prevention services recommended by the U.S. Preventive Services Task Force, but coverage of injectable PrEP is still inconsistent. Medi-Cal covers all PrEP methods in California, but this is not to case for all state Medicaid programs.
“If you’re uninsured and low income, you’re likely able to be eligible for ViiV’s patient assistance program. If you’re Medi-Cal eligible, you’re also able to access Apretude at no cost,” Roman said. “So the challenge has been for people who have private or commercial insurance. Medicare often is a challenge as well.”
Looking ahead to the availability of twice-yearly lenacapavir PrEP, Roman expects it to be “another great tool in the arsenal.” One advantage, he noted, is that it belongs to a different class of antiretrovirals, which decreases concern about resistance. Cabotegravir is an integrase inhibitor, a drug class widely used for HIV treatment. Lenacapavir, the first and only HIV capsid inhibitor, is currently only approved as part of a combination regimen for people with multidrug-resistant virus.
“Operationally, it’ll be great, because it limits the amount of time people need to come into the clinic,” Roman said. But that could present another challenge: “People who are engaging in frequent sex should be coming in and having routine sexual health visits. If people are only coming in twice a year for PrEP injections, hopefully they’ll come in more frequently for routine STI screening.”
PrEP Access for All
Having more HIV prevention options helps ensure that everyone can find a method that works for them. But to really change the game and contribute to ending the epidemic, these options must be available to all who need and want them.
To that end, SFAF is prioritizing equitable access to long-acting PrEP and “making sure these great options are getting into the right hands,” Roman said. “People who have higher resources are usually the ones who have access to these new interventions, so we always need to have an eye on folks who don’t typically have that option,” such as Black and Latino people, transgender individuals, lower-income people, unhoused people and sex workers. “We want to make sure that those folks are first in line for getting these new interventions, because those are the folks who are most impacted and disproportionately affected by HIV,” he added.
At the global level, advocates have been negotiating for years for wider access to Apretude in low- and middle-income countries, and they’re already demanding global access to lenacapavir, even before it’s been approved for PrEP. The drug (sold as Sunlenca) is currently priced at around $40,000 per year for HIV treatment, but a study presented at the AIDS conference showed that this could be brought down to around $40 with voluntary licensing and competition between generic suppliers.
Advocates say that reduced cost and improved access to long-acting PrEP will be necessary to reach global goals for eliminating HIV as a public health threat. “We are only six years away from 2030, and we still have 1.3 million new HIV infections per year,” said UNAIDS executive director Winnie Byanyima. “We want this miracle prevention to reach all those who need it now, not in six years’ time.”